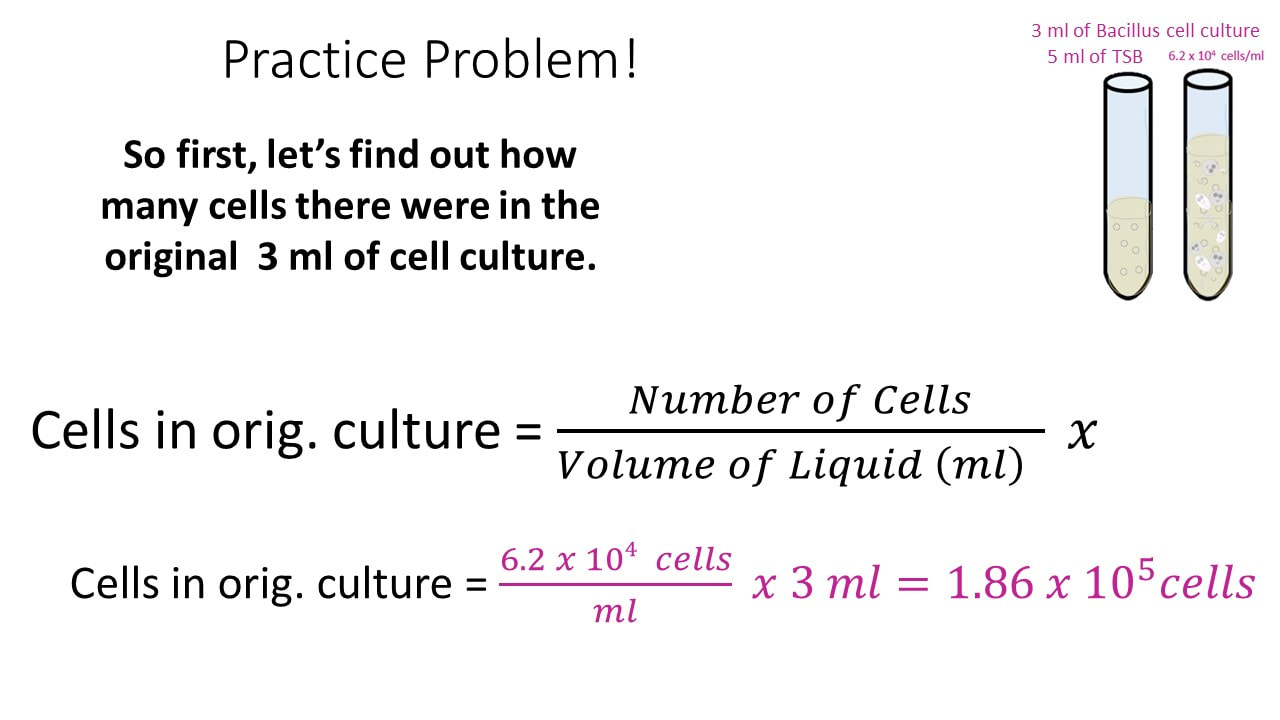
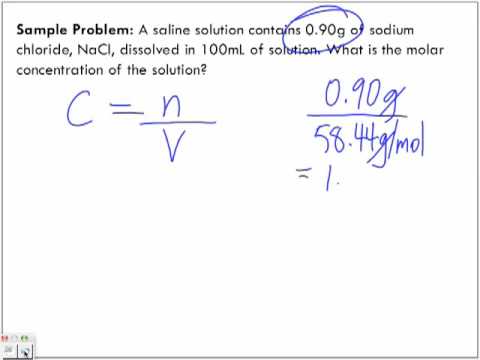
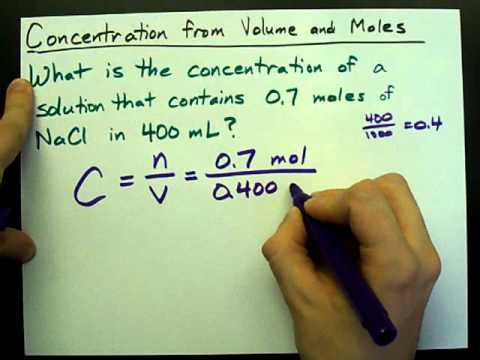
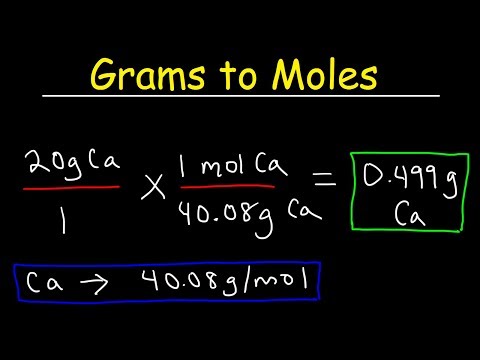
The concentration of the solution tells you how much solute has been dissolved in the solvent. There are many different units of concentration. The standard unit of concentration in chemistry is molarity . Molarity is defined as moles of solute per liters of solution.
It is important to note that molarity is per liters of solution, not per liters of solvent. For example, if one mole of salt were added to one liter of water, the resulting solution would not be 1 M (read as "one molar"). After the solution has been mixed, the volume would be recorded. The molarity could be calculated by dividing one mole by this total volume. How do chemists decide which units of concentration to use for a particular application? For many applications this may not be a problem, but for precise work these errors can become important.
In contrast, mole fraction, molality, and mass percentage depend on only the masses of the solute and solvent, which are independent of temperature. Many solutions contain one component, called the solvent, in which other components, called solutes, are dissolved. An aqueous solution is one for which the solvent is water.
The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. Concentrations may be measured using various units, with one very useful unit being molarity, defined as the number of moles of solute per liter of solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution.
For example, the known molecular weight of a chemical can be used along with the desired solution volume and solute concentration to determine the mass of chemical needed to make such a solution. An aqueous solution consists of at least two components, the solvent and the solute . Usually one wants to keep track of the amount of the solute dissolved in the solution. One could do by keeping track of the concentration by determining the mass of each component, but it is usually easier to measure liquids by volume instead of mass. To do this measure called molarity is commonly used.
Molarity is defined as the number of moles of solute divided by the volume of the solution in liters. To calculate the molarity of a solution, the number of moles of solute must be divided by the total liters of solution produced. To calculate molarity, divide the number of moles of solute by the volume of the solution in liters. Once you have the molar mass, multiply the number of grams of solute by 1 over the molar mass to convert the grams into moles.
Finally, divide the number of moles by the volume of the solution to get the molarity. There are several different ways to quantitatively describe the concentration of a solution. For example, molarity is a useful way to describe solution concentrations for reactions that are carried out in solution.
Mole fractions are used not only to describe gas concentrations but also to determine the vapor pressures of mixtures of similar liquids. Example \(\PageIndex\) reviews the methods for calculating the molarity and mole fraction of a solution when the masses of its components are known. Concentrations in chemistry are often measured in terms of moles per litre of solution. The mass of 1 mole is calculated using the chemical formula and the relative atomic masses of the elements. The number of moles present is found by dividing the mass by the mass of 1 mole. Concentrations are found by dividing the number of moles by the volume of solution, in litres.
Mole fraction is not very useful for experiments that involve quantitative reactions, but it is convenient for calculating the partial pressure of gases in mixtures, as discussed previously. Mole fractions are also useful for calculating the vapor pressures of certain types of solutions. Molality is particularly useful for determining how properties such as the freezing or boiling point of a solution vary with solute concentration. Units of ppb or ppm are also used to express very low concentrations, such as those of residual impurities in foods or of pollutants in environmental studies. While molality can be quite useful as a measurement of concentration, it isn't too convenient for converting to molarity. The reason is that we usually don't know how much volume the solute is going to occupy in the solution.
Some solutes even cause contraction of the solvent. For example, when 900 ml of distilled H2O is mixed with 100 ml of ethanol , the total volume of the resulting aqueous solution will be less than 1 liter. The ethanol molecules are capable of organizing H-bonded water molecules tightly around them, resulting in a smaller volume than the combined volumes of the separate components. It is important to note that the molarity is defined as moles of solute per liter of solution, not moles of solute per liter of solvent.
This is because when you add a substance, perhaps a salt, to some volume of water, the volume of the resulting solution will be different than the original volume in some unpredictable way. To get around this problem chemists commonly make up their solutions in volumetric flasks. These are flasks that have a long neck with an etched line indicating the volume. The solute is added to the flask first and then water is added until the solution reaches the mark.
The flasks have very good calibration so volumes are commonly known to at least four significant figures. Molality is an intensive property of solutions, and it is calculated as the moles of a solute divided by the kilograms of the solvent. Unlike molarity, which depends on the volume of the solution, molality depends only on the mass of the solvent. Since volume is subject to variation due to temperature and pressure, molarity also varies by temperature and pressure. In some cases, using weight is an advantage because mass does not vary with ambient conditions.
For example, molality is used when working with a range of temperatures. The units of molar concentration are moles per cubic decimeter. They are noted as mol/dm³ as well as M (pronounced "molar"). In many older books or articles, you can find different units of molar solutions - moles per liter (mol/l). Remember that one cubic decimeter equals to one liter, so these two notations express the same numeric values.
V is volume of solution in liters in which the indicated mass of solute must be dissolved to make the desired molar concentration . Note that V is the final or total volume of solution after the solute has been added to the solvent. The concentration of a substance is the quantity of solute present in a given quantity of solution. Concentrations are usually expressed as molarity, the number of moles of solute in 1 L of solution.
Figure 4.6 "Preparation of a Solution of Known Concentration Using a Solid Solute" illustrates this procedure for a solution of cobalt chloride dihydrate in ethanol. Note that the volume of the solvent is not specified. Because the solute occupies space in the solution, the volume of the solvent needed is almost always less than the desired volume of solution. For example, if the desired volume were 1.00 L, it would be incorrect to add 1.00 L of water to 342 g of sucrose because that would produce more than 1.00 L of solution. As shown in Figure 4.7 "Preparation of 250 mL of a Solution of (NH", for some substances this effect can be significant, especially for concentrated solutions. Concentrations are often reported on a mass-to-mass (m/m) basis or on a mass-to-volume (m/v) basis, particularly in clinical laboratories and engineering applications.
Each measurement can be expressed as a percentage by multiplying the ratio by 100; the result is reported as percent m/m or percent m/v. For aqueous solutions at 20°C, 1 ppm corresponds to 1 μg per milliliter, and 1 ppb corresponds to 1 ng per milliliter. These concentrations and their units are summarized in Table 4.1 "Common Units of Concentration". The labels on bottles of commercial reagents often describe the contents in terms of mass percentage. Sulfuric acid, for example, is sold as a 95% aqueous solution, or 95 g of \(\ce\) per 100 g of solution.
Parts per million and parts per billion are used to describe concentrations of highly dilute solutions. These measurements correspond to milligrams and micrograms of solute per kilogram of solution, respectively. For dilute aqueous solutions, this is equal to milligrams and micrograms of solute per liter of solution (assuming a density of 1.0 g/mL).
In many older books or articles, you can find different units of molar solutions – moles per liter (mol/l). This is a great chemistry tool for those who need to determine a solution's molar concentration from the mass, volume and molecular weight. There are four components in the equation therefore there are four fields in the molarity calculator. To compute any of the elements in the molarity equation you need to input the other three and choose the desired measurement unit. The student needs to be able to recognize the letter M as a substitute for the units moles solute/liter solution.
This is useful when considering chemical reactions that occur in solutions. In these reactions, a certain volume of solution one is added to another volume of solution two. The actual moles of each chemicals reacting depends on the molarity and the volume taken. This following discussion will focus on mass of solute/volume of solution and moles of solute/volume of solution concentration calculations.
Like normality, it is a unit of concentration in chemistry. Molarity is defined as the number of moles of solute per litre of solution. Molarity is often used in the calculation of pH i.e. dissociation or equilibrium constants, etc. Molarity is moles of solute divided by liters of solution, not solvent.
How To Calculate Volume From Number Of Moles Here we've just calculated an approximate molarity, but the volume effect of adding a small amount of solute to water is usually small, so this calculation probably isn't too bad. They are an important type of chemical calculation because when carrying out reactions, you need to know exactly how much of each chemical is being used if you are working with solutions. Many other types of calculations in chemistry involving solutions depend on getting these ones right. In an ionic solution, ionic strength is proportional to the sum of the molar concentration of salts. Both terms are used to express the concentration of a solution, but there is a significant difference between them. While molarity describes the amount of substance per unit volume of solution, molality defines the concentration as the amount of substance per unit mass of the solvent.
In other words, molality is the number of moles of solute per kilogram of solvent . This molarity calculator estimates the molar concentration of a solution by using the mass, volume and molecular weight. You can read more on the molar concentration and how to calculate the number of moles for a solution below the form. Molecules in a litre or even a cubic centimetre is enormous, it has become common practice to use what are called molar, rather than molecular, quantities.
A mole is the gram-molecular weight of a substance and, therefore, also Avogadro's number of molecules (6.02 × 1023). Thus, the number of moles in a sample is the weight of the sample divided by the molecular weight of the substance; it is also the number of molecules in the sample divided by Avogadro's number. Concentration in moles per litre (i.e., molarity) is usually designated by the letter M. Molar concentration is a measure of the concentration of a chemical species, in particular of a solute in a solution, in terms of amount of substance per unit volume of solution.
In chemistry, the most commonly used unit for molarity is the number of moles per liter, having the unit symbol mol/L or mol⋅dm−3 in SI unit. A solution with a concentration of 1 mol/L is said to be 1 molar, commonly designated as 1 M. To avoid confusion with SI prefix mega, which has the same abbreviation, small caps ᴍ or italicized M are also used in journals and textbooks. We then convert the number of moles of solute to the corresponding mass of solute needed.
Molarity is a unit of concentration, measuring the number of moles of a solute per liter of solution. The strategy for solving molarity problems is fairly simple. This outlines a straightforward method to calculate the molarity of a solution. So you are not confused with similar chemical terms, keep in mind that molarity means exactly the same as molar concentration . Molarity expresses the concentration of a solution.
It is defined as the number of moles of a substance or solute, dissolved per liter of solution (not per liter of solvent!). This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration . You can also calculate the mass of a substance needed to achieve a desired molarity. This article will provide you with the molarity definition and the molarity formula. Molarity is the number of moles of solute per liter of solution. A solute, which can be solid, liquid or gas, is a substance that is dissolved in a solvent.
The solvent is another substance that is capable of dissolving it within its intermolecular spaces. Together, the dissolved solute and the solvent make a solution. A typical solution is made by dissolving some solid substance in a liquid. The amount of substance that is dissolved in a given volume of liquid is known as the concentration of the liquid. Mathematically, concentration (\(c\)) is defined as moles of solute (\(n\)) per unit volume (\(V\)) of solution. The atomic weight of a substance is an assigned number which allows comparison of the relative masses of the different elements.
By definition, one atom of oxygen is assigned a "weight" of 16, and the atomic weights of the other elements are determined in relation to that of oxygen. In a molecule, i.e., a substance containing two or more different atoms, the molecular weight is equal to the sum of the atomic weights of the individual atoms. For example, the molecular weight of water is 18 ( + 16). In this explainer, we will learn how to calculate the molar concentration of a solution from the solvent volume and mass or moles of the dissolved solute. The molarity calculator calculates the mass of compound required to achieve a specific molar concentration and volume.
To dilute a solution of known molarity, please use the Solution Dilution Calculator. To dilute a solution of concentrated acid or base of known w/w% strength, please use the Acid & Base Molarity Calculator. The molar concentration unit [mol/ L ] is a conventionally widely used as concentration method.
It is the number of moles of target substance dissolved in 1 liter of solution. Solution concentrations are typically expressed as molarity and can be prepared by dissolving a known mass of solute in a solvent or diluting a stock solution. Table \(\PageIndex\) summarizes the different units of concentration and typical applications for each.